You are using an unsupported browser. Please upgrade your browser to a newer version to get the best experience on Human Metabolome Database.
| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-05-30 20:55:50 UTC |
|---|
| HMDB ID | HMDB0000482 |
|---|
| Secondary Accession Numbers | - HMDB00482
- HMDB0062511
- HMDB62511
|
|---|
| Metabolite Identification |
|---|
| Common Name | Caprylic acid |
|---|
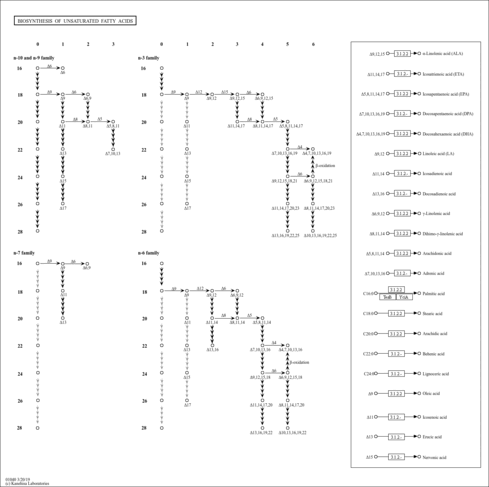
| Description | Caprylic acid is the common name for the eight-carbon straight-chain fatty acid known by the systematic name octanoic acid. It is found naturally in coconuts and breast milk. It is an oily liquid with a slightly unpleasant rancid taste that is minimally soluble in water. Caprylic acid is used commercially in the production of esters used in perfumery and also in the manufacture of dyes (Wikipedia ). Caprylic acid can be found in numerous foods such as Prunus (Cherry, Plum), pineapple sages, black raspberries, and shallots. Caprylic acid is found to be associated with medium-chain acyl-CoA dehydrogenase deficiency, which is an inborn error of metabolism. |
|---|
| Structure | InChI=1S/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10) |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Heptanecarboxylic acid | ChEBI | | 8:0 | ChEBI | | Acide octanoique | ChEBI | | Acido octanoico | ChEBI | | Acidum octanocium | ChEBI | | Acidum octanoicum | ChEBI | | C8:0 | ChEBI | | CH3-[CH2]6-COOH | ChEBI | | Kaprylsaeure | ChEBI | | N-Caprylic acid | ChEBI | | N-Octanoic acid | ChEBI | | N-Octoic acid | ChEBI | | N-Octylic acid | ChEBI | | OCTANOIC ACID (caprylIC ACID) | ChEBI | | Octansaeure | ChEBI | | Octoic acid | ChEBI | | Octylic acid | ChEBI | | Octanoate | Kegg | | 1-Heptanecarboxylate | Generator | | N-Caprylate | Generator | | N-Octanoate | Generator | | N-Octoate | Generator | | N-Octylate | Generator | | OCTANOate (caprylate) | Generator | | Octoate | Generator | | Octylate | Generator | | Octanoic acid | Generator | | Caprylate | Generator | | Emery 657 | HMDB | | Kortacid 0899 | HMDB | | Lunac 8-95 | HMDB | | Lunac 8-98 | HMDB | | Neo-fat 8 | HMDB | | Neo-fat 8S | HMDB | | Prifac 2901 | HMDB | | Caprylic acid, cadmium salt | HMDB | | Caprylic acid, cesium salt | HMDB | | Caprylic acid, manganese salt | HMDB | | Caprylic acid, nickel(+2) salt | HMDB | | Caprylic acid, zinc salt | HMDB | | Caprylic acid, aluminum salt | HMDB | | Caprylic acid, barium salt | HMDB | | Caprylic acid, chromium(+2) salt | HMDB | | Caprylic acid, lead(+2) salt | HMDB | | Caprylic acid, potassium salt | HMDB | | Caprylic acid, tin(+2) salt | HMDB | | Sodium octanoate | HMDB | | Caprylic acid, 14C-labeled | HMDB | | Caprylic acid, lithium salt | HMDB | | Caprylic acid, ruthenium(+3) salt | HMDB | | Caprylic acid, sodium salt | HMDB | | Caprylic acid, sodium salt, 11C-labeled | HMDB | | Caprylic acid, tin salt | HMDB | | Caprylic acid, zirconium salt | HMDB | | Sodium caprylate | HMDB | | Caprylic acid, ammonia salt | HMDB | | Caprylic acid, calcium salt | HMDB | | Caprylic acid, cobalt salt | HMDB | | Caprylic acid, copper salt | HMDB | | Caprylic acid, copper(+2) salt | HMDB | | Caprylic acid, iridum(+3) salt | HMDB | | Caprylic acid, iron(+3) salt | HMDB | | Caprylic acid, lanthanum(+3) salt | HMDB | | Caprylic acid, zirconium(+4) salt | HMDB | | FA(8:0) | HMDB | | Lithium octanoate | HMDB |
|
|---|
| Chemical Formula | C8H16O2 |
|---|
| Average Molecular Weight | 144.2114 |
|---|
| Monoisotopic Molecular Weight | 144.115029756 |
|---|
| IUPAC Name | octanoic acid |
|---|
| Traditional Name | caprylic acid |
|---|
| CAS Registry Number | 124-07-2 |
|---|
| SMILES | CCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10) |
|---|
| InChI Key | WWZKQHOCKIZLMA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | Not Available |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 16.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.79 mg/mL | Not Available | | LogP | 3.05 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Caprylic acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0159-0910000000-e609d5de69ede6fbcde0 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid GC-MS (1 TMS) | splash10-0gb9-1920000000-1dc6ed976e003f99e23d | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid EI-B (Non-derivatized) | splash10-03kc-9000000000-74cfce03769b41313952 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid EI-B (Non-derivatized) | splash10-0uyi-0931100000-7668e36e436054042b5c | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid GC-EI-TOF (Non-derivatized) | splash10-0159-0910000000-e609d5de69ede6fbcde0 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid GC-MS (Non-derivatized) | splash10-0gb9-1920000000-1dc6ed976e003f99e23d | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Caprylic acid GC-EI-TOF (Non-derivatized) | splash10-014i-0910000000-9ecb4ccb46e89710f76a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Caprylic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0596-9100000000-8854c56056d8d3405087 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Caprylic acid GC-MS (1 TMS) - 70eV, Positive | splash10-062l-9100000000-67da39159980b9078fab | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Caprylic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Caprylic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03kc-9000000000-8c7db9a9d75b2cc1bd1c | 2014-09-20 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid Quattro_QQQ 10V, Negative-QTOF (Annotated) | splash10-0006-0900000000-bb237630bedcee6145d1 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid Quattro_QQQ 25V, Negative-QTOF (Annotated) | splash10-0006-2900000000-98284f50a271878c8481 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid Quattro_QQQ 40V, Negative-QTOF (Annotated) | splash10-0006-5900000000-209fbbacd4996b9a770d | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid EI-B (HITACHI M-80B) , Positive-QTOF | splash10-03kc-9000000000-74cfce03769b41313952 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-0006-0900000000-e2c4dff10393086340f7 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-0006-1900000000-bbde8564b4e5543a636a | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid DI-ESI-Q-Exactive Plus , Negative-QTOF | splash10-0006-0900000000-d219a0c6559a4783fd81 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid LC-ESI-QQ , negative-QTOF | splash10-0006-0900000000-e2c4dff10393086340f7 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid LC-ESI-QQ , negative-QTOF | splash10-0006-1900000000-bbde8564b4e5543a636a | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid LC-ESI-IT , negative-QTOF | splash10-0006-0900000000-ca22ecd0b25fa371fc73 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid 40V, Negative-QTOF | splash10-0gvp-9100000000-58c00a2024ba0bae4ff4 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid 20V, Negative-QTOF | splash10-0002-9300000000-2505e6738cf5d5877654 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Caprylic acid 10V, Negative-QTOF | splash10-0006-0900000000-1ab4cdf8f74c93312743 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 10V, Positive-QTOF | splash10-002b-2900000000-ac7c80e50c93b8cc3af7 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 20V, Positive-QTOF | splash10-0002-9400000000-d2694b5f89d8eaeb2ab1 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 40V, Positive-QTOF | splash10-052f-9000000000-f2b24072c91d8b0e4078 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 10V, Negative-QTOF | splash10-0006-1900000000-ad62d44e72f4dd1760ab | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 20V, Negative-QTOF | splash10-0007-7900000000-9c869b84815f21dffc7c | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 40V, Negative-QTOF | splash10-0a4l-9000000000-b8a648d443695914e479 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 10V, Positive-QTOF | splash10-0a6u-9100000000-f5c38fdad70b324e75ac | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 20V, Positive-QTOF | splash10-0a4i-9000000000-bee103956686b4840d1e | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 40V, Positive-QTOF | splash10-0a4l-9000000000-e7cbd036c8e80018c825 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 10V, Negative-QTOF | splash10-0006-0900000000-8f2b048da6331570976c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 20V, Negative-QTOF | splash10-0006-0900000000-cd31ca01a5fa6be86acb | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Caprylic acid 40V, Negative-QTOF | splash10-052f-9100000000-682c215be5abe1fe87ed | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane (predicted from logP)
|
|---|
| Biospecimen Locations | - Blood
- Breast Milk
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Sweat
- Urine
|
|---|
| Tissue Locations | |
|---|
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected and Quantified | 2.5 (0.0-5.0) uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 8.0 (5.0-19.0) uM | Adult (>18 years old) | Both | Normal | | details | | Breast Milk | Detected and Quantified | 267 +/- 154 uM | Adult (>18 years old) | Female | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 2.50 +/- 2.50 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.738 +/- 0.193 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.741 +/- 0.162 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 0.218 +/- 0.635 uM | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.629 +/- 0.249 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Sweat | Detected but not Quantified | Not Quantified | Adult | Both | Normal | | details | | Urine | Detected and Quantified | 0.39 +/- 0.37 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.15 (0.04-0.22) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.0 umol/mmol creatinine | Children (1 - 18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 4-10 umol/mmol creatinine | Children (1 - 13 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Crohns disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Ulcerative colitis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Crohn's disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Ulcerative colitis | | details | | Feces | Detected but not Quantified | Not Quantified | Children (1-13 years old) | Both | Autism | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Breast cancer | | details | | Urine | Detected and Quantified | 16-410 umol/mmol creatinine | Children (1 - 13 years old) | Both | Medium Chain Acyl-CoA Dehydrogenase Deficiency | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Autism |
|---|
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R: Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013 Oct 9;8(10):e76993. doi: 10.1371/journal.pone.0076993. eCollection 2013. [PubMed:24130822 ]
| | Crohn's disease |
|---|
- Ahmed I, Greenwood R, Costello B, Ratcliffe N, Probert CS: Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Aliment Pharmacol Ther. 2016 Mar;43(5):596-611. doi: 10.1111/apt.13522. Epub 2016 Jan 25. [PubMed:26806034 ]
- De Preter V, Machiels K, Joossens M, Arijs I, Matthys C, Vermeire S, Rutgeerts P, Verbeke K: Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut. 2015 Mar;64(3):447-58. doi: 10.1136/gutjnl-2013-306423. Epub 2014 May 8. [PubMed:24811995 ]
| | Ulcerative colitis |
|---|
- Ahmed I, Greenwood R, Costello B, Ratcliffe N, Probert CS: Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Aliment Pharmacol Ther. 2016 Mar;43(5):596-611. doi: 10.1111/apt.13522. Epub 2016 Jan 25. [PubMed:26806034 ]
- De Preter V, Machiels K, Joossens M, Arijs I, Matthys C, Vermeire S, Rutgeerts P, Verbeke K: Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut. 2015 Mar;64(3):447-58. doi: 10.1136/gutjnl-2013-306423. Epub 2014 May 8. [PubMed:24811995 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Perillyl alcohol administration for cancer treatment |
|---|
- Silva CL, Passos M, Camara JS: Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers--a powerful strategy for breast cancer diagnosis. Talanta. 2012 Jan 30;89:360-8. doi: 10.1016/j.talanta.2011.12.041. Epub 2011 Dec 22. [PubMed:22284503 ]
| | Medium Chain Acyl-CoA Dehydrogenase Deficiency |
|---|
- Gregersen N, Kolvraa S, Rasmussen K, Mortensen PB, Divry P, David M, Hobolth N: General (medium-chain) acyl-CoA dehydrogenase deficiency (non-ketotic dicarboxylic aciduria): quantitative urinary excretion pattern of 23 biologically significant organic acids in three cases. Clin Chim Acta. 1983 Aug 15;132(2):181-91. [PubMed:6616873 ]
|
|
|---|
| Associated OMIM IDs | - 209850 (Autism)
- 266600 (Crohn's disease)
- 114500 (Colorectal cancer)
- 201450 (Medium Chain Acyl-CoA Dehydrogenase Deficiency)
|
|---|
| External Links |
|---|
| DrugBank ID | DB04519 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB003336 |
|---|
| KNApSAcK ID | C00001231 |
|---|
| Chemspider ID | 370 |
|---|
| KEGG Compound ID | C06423 |
|---|
| BioCyc ID | CPD-195 |
|---|
| BiGG ID | 48223 |
|---|
| Wikipedia Link | Caprylic_acid |
|---|
| METLIN ID | 5469 |
|---|
| PubChem Compound | 379 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 28837 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | OCTA |
|---|
| MarkerDB ID | MDB00000171 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Caprylic acid. Jpn. Kokai Tokkyo Koho (1983), 4 pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL: Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648-69. [PubMed:8412012 ]
- Giannakou SA, Dallas PP, Rekkas DM, Choulis NH: In vitro evaluation of nimodipine permeation through human epidermis using response surface methodology. Int J Pharm. 2002 Jul 8;241(1):27-34. [PubMed:12086718 ]
- Dieterle F, Muller-Hagedorn S, Liebich HM, Gauglitz G: Urinary nucleosides as potential tumor markers evaluated by learning vector quantization. Artif Intell Med. 2003 Jul;28(3):265-79. [PubMed:12927336 ]
- Nair MK, Joy J, Venkitanarayanan KS: Inactivation of Enterobacter sakazakii in reconstituted infant formula by monocaprylin. J Food Prot. 2004 Dec;67(12):2815-9. [PubMed:15633694 ]
- Habeeb AF, Francis RD: Preparation of human immunoglobulin by caprylic acid precipitation. Prep Biochem. 1984;14(1):1-17. [PubMed:6718324 ]
- Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
|
|---|