| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:42 UTC |
|---|
| HMDB ID | HMDB0001431 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pyridoxamine |
|---|
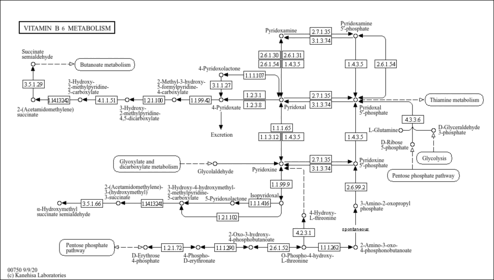
| Description | Pyridoxamine is one form of vitamin B6. Chemically it is based on a pyridine ring structure, with hydroxyl, methyl, aminomethyl, and hydroxymethyl substituents. It differs from pyridoxine by the substituent at the 4-position. The hydroxyl at position 3 and aminomethyl group at position 4 of its ring endow pyridoxamine with a variety of chemical properties, including the scavenging of free radical species and carbonyl species formed in sugar and lipid degradation and chelation of metal ions that catalyze Amadori reactions. Pyridoxamine, also known as PM, belongs to the class of organic compounds known as pyridoxamine 5'-phosphates. These are heterocyclic aromatic compounds containing a pyridoxamine that carries a phosphate group at the 5'-position. Within humans, pyridoxamine participates in a number of enzymatic reactions. In particular, pyridoxamine can be converted into pyridoxal; which is mediated by the enzyme pyridoxine-5'-phosphate oxidase. In addition, pyridoxamine can be converted into pyridoxamine 5'-phosphate; which is catalyzed by the enzyme pyridoxal kinase. Pyridoxamine also inhibits the formation of advanced lipoxidation endproducts during lipid peroxidation reactions by reaction with dicarbonyl intermediates. In humans, pyridoxamine is involved in vitamin B6 metabolism. Outside of the human body, pyridoxamine has been detected, but not quantified in several different foods, such as nutmegs, sparkleberries, fennels, turmerics, and swiss chards. Pyridoxamine inhibits the Maillard reaction and can block the formation of advanced glycation endproducts, which are associated with medical complications of diabetes. Pyridoxamine is hypothesized to trap intermediates in the formation of Amadori products released from glycated proteins, possibly preventing the breakdown of glycated proteins by disrupting the catalysis of this process through disruptive interactions with the metal ions crucial to the redox reaction. One research study found that pyridoxamine specifically reacts with the carbonyl group in Amadori products, but inhibition of post-Amadori reactions (that can lead to advanced glycation endproducts) is due in much greater part to the metal chelation effects of pyridoxamine. |
|---|
| Structure | InChI=1S/C8H12N2O2/c1-5-8(12)7(2-9)6(4-11)3-10-5/h3,11-12H,2,4,9H2,1H3 |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(AMINOMETHYL)-5-(hydroxymethyl)-2-methylpyridin-3-ol | ChEBI | | PM | ChEBI | | 2-Methyl-4-aminomethyl-5-hydroxymethyl-3-pyridinol | HMDB | | 4-(Aminomethyl)-5-(hydroxymethyl)-2-methyl-3-pyridinol | HMDB | | 4-(Aminomethyl)-5-hydroxy-6-methyl-3-pyridinemethanol | HMDB | | Pyridoxylamine | HMDB |
|
|---|
| Chemical Formula | C8H12N2O2 |
|---|
| Average Molecular Weight | 168.1931 |
|---|
| Monoisotopic Molecular Weight | 168.089877638 |
|---|
| IUPAC Name | 4-(aminomethyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol |
|---|
| Traditional Name | pyridoxamine |
|---|
| CAS Registry Number | 85-87-0 |
|---|
| SMILES | CC1=C(O)C(CN)=C(CO)C=N1 |
|---|
| InChI Identifier | InChI=1S/C8H12N2O2/c1-5-8(12)7(2-9)6(4-11)3-10-5/h3,11-12H,2,4,9H2,1H3 |
|---|
| InChI Key | NHZMQXZHNVQTQA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyridoxamine 5'-phosphates. These are heterocyclic aromatic compounds containing a pyridoxamine that carries a phosphate group at the 5'-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridoxamines |
|---|
| Direct Parent | Pyridoxamine 5'-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoxamine 5'-phosphate
- Aralkylamine
- Hydroxypyridine
- Methylpyridine
- Heteroaromatic compound
- Azacycle
- Amine
- Hydrocarbon derivative
- Alcohol
- Aromatic alcohol
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 815 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Pyridoxamine,1TMS,isomer #1 | CC1=NC=C(CO)C(CN)=C1O[Si](C)(C)C | 1773.5 | Semi standard non polar | 33892256 | | Pyridoxamine,1TMS,isomer #2 | CC1=NC=C(CO[Si](C)(C)C)C(CN)=C1O | 1806.8 | Semi standard non polar | 33892256 | | Pyridoxamine,1TMS,isomer #3 | CC1=NC=C(CO)C(CN[Si](C)(C)C)=C1O | 1931.6 | Semi standard non polar | 33892256 | | Pyridoxamine,2TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN)=C1O[Si](C)(C)C | 1836.4 | Semi standard non polar | 33892256 | | Pyridoxamine,2TMS,isomer #2 | CC1=NC=C(CO)C(CN[Si](C)(C)C)=C1O[Si](C)(C)C | 1905.4 | Semi standard non polar | 33892256 | | Pyridoxamine,2TMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C)C(CN[Si](C)(C)C)=C1O | 1940.8 | Semi standard non polar | 33892256 | | Pyridoxamine,2TMS,isomer #4 | CC1=NC=C(CO)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O | 2050.6 | Semi standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN[Si](C)(C)C)=C1O[Si](C)(C)C | 1960.1 | Semi standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN[Si](C)(C)C)=C1O[Si](C)(C)C | 1980.0 | Standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN[Si](C)(C)C)=C1O[Si](C)(C)C | 2036.3 | Standard polar | 33892256 | | Pyridoxamine,3TMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2037.0 | Semi standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2109.5 | Standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2182.8 | Standard polar | 33892256 | | Pyridoxamine,3TMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O | 2056.0 | Semi standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O | 2126.9 | Standard non polar | 33892256 | | Pyridoxamine,3TMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O | 2196.2 | Standard polar | 33892256 | | Pyridoxamine,4TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2103.3 | Semi standard non polar | 33892256 | | Pyridoxamine,4TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2102.9 | Standard non polar | 33892256 | | Pyridoxamine,4TMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C)C(CN([Si](C)(C)C)[Si](C)(C)C)=C1O[Si](C)(C)C | 2012.5 | Standard polar | 33892256 | | Pyridoxamine,1TBDMS,isomer #1 | CC1=NC=C(CO)C(CN)=C1O[Si](C)(C)C(C)(C)C | 2057.3 | Semi standard non polar | 33892256 | | Pyridoxamine,1TBDMS,isomer #2 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN)=C1O | 2067.2 | Semi standard non polar | 33892256 | | Pyridoxamine,1TBDMS,isomer #3 | CC1=NC=C(CO)C(CN[Si](C)(C)C(C)(C)C)=C1O | 2209.0 | Semi standard non polar | 33892256 | | Pyridoxamine,2TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN)=C1O[Si](C)(C)C(C)(C)C | 2339.2 | Semi standard non polar | 33892256 | | Pyridoxamine,2TBDMS,isomer #2 | CC1=NC=C(CO)C(CN[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2393.3 | Semi standard non polar | 33892256 | | Pyridoxamine,2TBDMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN[Si](C)(C)C(C)(C)C)=C1O | 2406.1 | Semi standard non polar | 33892256 | | Pyridoxamine,2TBDMS,isomer #4 | CC1=NC=C(CO)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O | 2470.7 | Semi standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2620.3 | Semi standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2570.1 | Standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2421.4 | Standard polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2684.2 | Semi standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2681.5 | Standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #2 | CC1=NC=C(CO)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2495.8 | Standard polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O | 2686.0 | Semi standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O | 2689.5 | Standard non polar | 33892256 | | Pyridoxamine,3TBDMS,isomer #3 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O | 2506.3 | Standard polar | 33892256 | | Pyridoxamine,4TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2945.2 | Semi standard non polar | 33892256 | | Pyridoxamine,4TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2828.6 | Standard non polar | 33892256 | | Pyridoxamine,4TBDMS,isomer #1 | CC1=NC=C(CO[Si](C)(C)C(C)(C)C)C(CN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=C1O[Si](C)(C)C(C)(C)C | 2477.6 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-001j-0590000000-a4bf9fe291241c903e3d | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9350000000-3b1415672e7ab22d5e64 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-MS (3 TMS) | splash10-001i-1490000000-7214a8743cbe260268ce | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-EI-TOF (Non-derivatized) | splash10-001j-0590000000-a4bf9fe291241c903e3d | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-EI-TOF (Non-derivatized) | splash10-00di-9350000000-3b1415672e7ab22d5e64 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Pyridoxamine GC-MS (Non-derivatized) | splash10-001i-1490000000-7214a8743cbe260268ce | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxamine GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-2900000000-1dfbec04a6ae497e3229 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxamine GC-MS (2 TMS) - 70eV, Positive | splash10-00dj-6090000000-1aab191c7ca62559a838 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxamine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyridoxamine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-014i-0900000000-4ca97fcd886d80336a5c | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-001i-1900000000-499c48bce709456248bc | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0059-9200000000-aee74b4498be7c8cea6f | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-014i-0900000000-f1da14360063a04c11b4 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-014r-0900000000-f5aea0fa928eb266a79d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-00di-0900000000-bebad702dbdba57d61fa | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-05fr-0900000000-7322846ca1b4d95b5b16 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0ab9-2900000000-da43b1a049d349d91831 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-014i-0900000000-6b5f1d606df91ae52eb4 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive-QTOF | splash10-0gb9-0900000000-d5a16a4cdc19453ae9c7 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-01b9-0900000000-3a1f853ce36bb047e05f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ , negative-QTOF | splash10-014i-0900000000-f1da14360063a04c11b4 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ , negative-QTOF | splash10-014r-0900000000-f5aea0fa928eb266a79d | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ , negative-QTOF | splash10-00di-0900000000-c7a2dec0f7555dcc5ef7 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ , negative-QTOF | splash10-05fr-0900000000-fe3f1f78fad714162a1c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QQ , negative-QTOF | splash10-0ab9-2900000000-da43b1a049d349d91831 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QTOF , negative-QTOF | splash10-01b9-0900000000-f0cc74a9651686c1610c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine , negative-QTOF | splash10-014i-0900000000-9303a95b8f51c5ce6c22 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyridoxamine LC-ESI-QTOF , positive-QTOF | splash10-014i-0900000000-6b5f1d606df91ae52eb4 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 10V, Positive-QTOF | splash10-0uxr-0900000000-a1db882596b08c2987a7 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 20V, Positive-QTOF | splash10-0ue9-0900000000-b0f20e8503b963c8a31f | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 40V, Positive-QTOF | splash10-001i-3900000000-b41efa32a572817ef0c9 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 10V, Negative-QTOF | splash10-014i-0900000000-cd842fcc776d552a0f07 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 20V, Negative-QTOF | splash10-052r-0900000000-ae76358c88e1a1cf17dc | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyridoxamine 40V, Negative-QTOF | splash10-0596-9600000000-eeec53c65ada2bc944b0 | 2015-05-27 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Esteve-Romero J, Capella-Peiro ME, Monferrer-Pons L, Gil-Agusti M: Micellar liquid chromatography in clinical chemistry: application to the monitorization of B6 vitamins. Clin Chim Acta. 2004 Oct;348(1-2):69-77. [PubMed:15369738 ]
- Berzas Nevado JJ, Murillo Pulgarin JA, Gomez Laguna MA: Determination of pyridoxamine in urine by matrix isopotential synchronous fluorescence spectrometry. Analyst. 1995 Jan;120(1):171-4. [PubMed:7710125 ]
- Sharma SK, Dakshinamurti K: Determination of vitamin B6 vitamers and pyridoxic acid in biological samples. J Chromatogr. 1992 Jul 1;578(1):45-51. [PubMed:1400785 ]
- Rokitzki L, Sagredos AN, Reuss F, Buchner M, Keul J: Acute changes in vitamin B6 status in endurance athletes before and after a marathon. Int J Sport Nutr. 1994 Jun;4(2):154-65. [PubMed:8054960 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|