| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-05-30 20:55:54 UTC |
|---|
| HMDB ID | HMDB0000134 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Fumaric acid |
|---|
| Description | Fumaric acid is a dicarboxylic acid. It is a precursor to L-malate in the Krebs tricarboxylic acid (TCA) cycle. It is formed by the oxidation of succinic acid by succinate dehydrogenase. Fumarate is converted by the enzyme fumarase to malate. Fumaric acid has recently been identified as an oncometabolite or an endogenous, cancer causing metabolite. High levels of this organic acid can be found in tumors or biofluids surrounding tumors. Its oncogenic action appears to due to its ability to inhibit prolyl hydroxylase-containing enzymes. In many tumours, oxygen availability becomes limited (hypoxia) very quickly due to rapid cell proliferation and limited blood vessel growth. The major regulator of the response to hypoxia is the HIF transcription factor (HIF-alpha). Under normal oxygen levels, protein levels of HIF-alpha are very low due to constant degradation, mediated by a series of post-translational modification events catalyzed by the prolyl hydroxylase domain-containing enzymes PHD1, 2 and 3, (also known as EglN2, 1 and 3) that hydroxylate HIF-alpha and lead to its degradation. All three of the PHD enzymes are inhibited by fumarate. Fumaric acid is found to be associated with fumarase deficiency, which is an inborn error of metabolism. It is also a metabolite of Aspergillus. |
|---|
| Structure | InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ |
|---|
| Synonyms | | Value | Source |
|---|
| (2E)-2-Butenedioic acid | ChEBI | | (e)-2-Butenedioic acid | ChEBI | | e297 | ChEBI | | Fumarsaeure | ChEBI | | trans-1,2-Ethylenedicarboxylic acid | ChEBI | | trans-But-2-enedioic acid | ChEBI | | trans-Butenedioic acid | ChEBI | | (2E)-2-Butenedioate | Generator | | (e)-2-Butenedioate | Generator | | trans-1,2-Ethylenedicarboxylate | Generator | | trans-But-2-enedioate | Generator | | trans-Butenedioate | Generator | | Fumarate | Generator | | (2E)-But-2-enedioate | HMDB | | (2E)-But-2-enedioic acid | HMDB | | 2-(e)-Butenedioate | HMDB | | 2-(e)-Butenedioic acid | HMDB | | Allomaleate | HMDB | | Allomaleic acid | HMDB | | Boletate | HMDB | | Boletic acid | HMDB | | FC 33 | HMDB | | Lichenate | HMDB | | Lichenic acid | HMDB | | trans-2-Butenedioate | HMDB | | trans-2-Butenedioic acid | HMDB | | Furamag | HMDB | | Mafusol | HMDB | | Fumaric acid | HMDB |
| Show more...
|---|
| Chemical Formula | C4H4O4 |
|---|
| Average Molecular Weight | 116.0722 |
|---|
| Monoisotopic Molecular Weight | 116.010958616 |
|---|
| IUPAC Name | (2E)-but-2-enedioic acid |
|---|
| Traditional Name | fumaric acid |
|---|
| CAS Registry Number | 110-17-8 |
|---|
| SMILES | OC(=O)\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ |
|---|
| InChI Key | VZCYOOQTPOCHFL-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl
- Fatty acid
- Unsaturated fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | Biological locationRoute of exposureSourceExogenous- Exogenous (HMDB: HMDB0000134)
Food- Food (HMDB: HMDB0000134)
Animal originBeverageFruitSoyVegetableHerb and spiceMilk and milk productUnfermented milk- Milk (Cow) (FooDB: FOOD00618)
- Cow milk, pasteurized, vitamin A + D added, 0% fat (FooDB: FOOD00889)
- Cow milk, pasteurized, vitamin A + D added, 1% fat (FooDB: FOOD00890)
- Cow milk, pasteurized, vitamin A + D added, 2% fat (FooDB: FOOD00891)
- Cow milk, pasteurized, vitamin D added, 3.25% fat (FooDB: FOOD00892)
PulseAquatic origin
Endogenous |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 549 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 7 mg/mL | Not Available | | LogP | 0.46 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized | Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0002-2940000000-e988056514d4ce4acc27 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0002-2960000000-a5ebaf2bbade922838ec | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-0002-2950000000-32afa4d45e0e72b174b4 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0002-0950000000-fe0f05c02c783d0b6f6b | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-006t-9530000000-0fc03f31f09dc8dbf4c6 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-MS (2 TMS) | splash10-0002-3690000000-75089756992cdbe841e3 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid EI-B (Non-derivatized) | splash10-0002-9100000000-2cf649749b42cc0c610c | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid EI-B (Non-derivatized) | splash10-0007-0890000000-b1c35cd55deb81254f66 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-0002-2940000000-e988056514d4ce4acc27 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-0002-2960000000-a5ebaf2bbade922838ec | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-0002-2950000000-32afa4d45e0e72b174b4 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-0002-0950000000-fe0f05c02c783d0b6f6b | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-006t-9530000000-0fc03f31f09dc8dbf4c6 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-MS (Non-derivatized) | splash10-0002-3690000000-75089756992cdbe841e3 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Fumaric acid GC-EI-TOF (Non-derivatized) | splash10-0002-0940000000-177fdb9168659029ffaa | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ba-9200000000-52f88e04bac0ff8cdf17 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (2 TMS) - 70eV, Positive | splash10-00di-8920000000-06da44f348d0fe0358b3 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Fumaric acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0092-9000000000-003dd2d9303272b2ebea | 2014-09-20 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid Quattro_QQQ 10V, Negative-QTOF (Annotated) | splash10-00di-9100000000-57f13cd433a6fe4bf0b3 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid Quattro_QQQ 25V, Negative-QTOF (Annotated) | splash10-0229-9600000000-cd9e2979d0bb1e2a62f2 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid Quattro_QQQ 40V, Negative-QTOF (Annotated) | splash10-03k9-8900000000-dc50dbf8a50872383d54 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid EI-B (HITACHI RMU-6L) , Positive-QTOF | splash10-0002-9100000000-b47e534bc82a6ed36e7c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 10V, Negative-QTOF | splash10-00di-9000000000-04b277f233e1bd56c9af | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 10V, Negative-QTOF | splash10-03di-4900000000-99764eff7d7cb7bfaee3 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 35V, Negative-QTOF | splash10-00di-9000000000-453b921a0aaf64cfd558 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 40V, Positive-QTOF | splash10-0f6x-9000000000-cd7e7026d13bb5fe844b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 10V, Positive-QTOF | splash10-00dj-9000000000-383c22a29e2769626c47 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 20V, Positive-QTOF | splash10-0ir3-9000000000-3013847e2e0f9bf18d29 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 30V, Negative-QTOF | splash10-00di-9000000000-9cc4f29b523ed34dc0b6 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 15V, Negative-QTOF | splash10-00di-9000000000-d9885a6093f38dd8b9fa | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 45V, Negative-QTOF | splash10-00di-9000000000-46fdee94b5a787f1b81f | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 35V, Negative-QTOF | splash10-03k9-8900000000-3ff0fb725ec9dffc3688 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Fumaric acid 20V, Negative-QTOF | splash10-014m-9000000000-394fd8f1b59c7befec9d | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 10V, Positive-QTOF | splash10-014j-9800000000-dbf34563376daeb2901f | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 20V, Positive-QTOF | splash10-00xs-9200000000-113bf08cdc77707ed3ad | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 40V, Positive-QTOF | splash10-00fr-9000000000-f32d7ec65e8649bc2b0a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 10V, Negative-QTOF | splash10-014i-2900000000-408d53a9fff7acc8ca23 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 20V, Negative-QTOF | splash10-014i-6900000000-f149e9e5a34e27dd4d4e | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 40V, Negative-QTOF | splash10-0fxt-9000000000-cae5f71dc27daaf17d9e | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 10V, Negative-QTOF | splash10-00di-9000000000-3d34cd791255c022876a | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 20V, Negative-QTOF | splash10-00di-9000000000-55a5c43ec34fdd2cb0a4 | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 40V, Negative-QTOF | splash10-0uk9-9000000000-7c9639a65d534cd8ff97 | 2021-09-21 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Fumaric acid 10V, Positive-QTOF | splash10-000t-9000000000-c5eb63dc643f72e91ede | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | - Extracellular
- Membrane
- Mitochondria
|
|---|
| Biospecimen Locations | - Blood
- Breast Milk
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Sweat
- Urine
|
|---|
| Tissue Locations | |
|---|
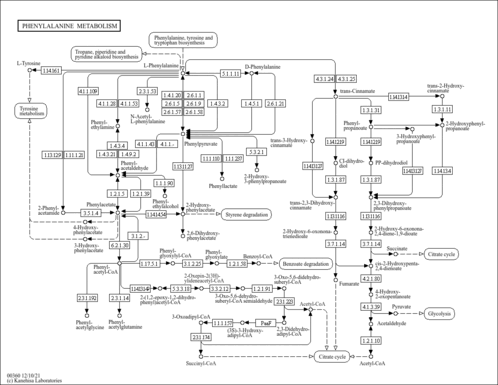
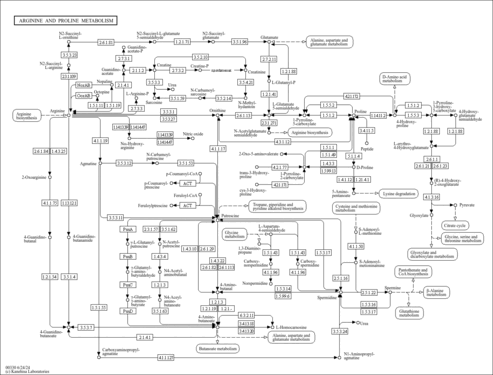
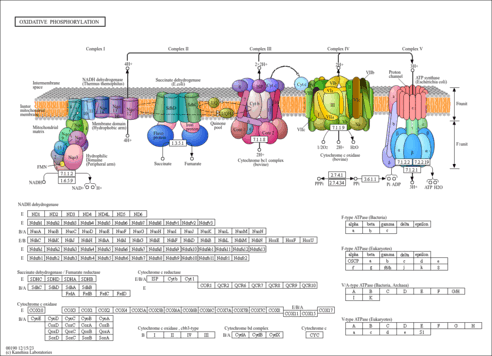
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1.5 (0.0-4.0) uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.49-1.4 uM | Infant (0-1 year old) | Both | Normal | | details | | Breast Milk | Detected and Quantified | 2.4 +/- 1.5 uM | Adult (>18 years old) | Female | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 1 uM | Children (1-13 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 33.0 (9.00-57.0) uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected and Quantified | 84.430 +/- 50.830 nmol/g wet feces | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.707 +/- 0.303 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.778 +/- 1.07 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.798 +/- 0.396 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 0.997 +/- 0.492 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected and Quantified | 1.95 +/- 0.66 uM | Adult (>18 years old) | Both | Normal | | details | | Sweat | Detected and Quantified | 10-40 uM | Adult (60 years old) | Male | Normal | | details | | Sweat | Detected and Quantified | 10 uM | Adult (40 years old) | Male | Normal | | details | | Sweat | Detected but not Quantified | Not Quantified | Adult | Both | Normal | | details | | Urine | Detected and Quantified | 0-1.5 umol/mmol creatinine | Infant (0-1 year old) | Female | Normal | | details | | Urine | Detected and Quantified | <14 umol/mmol creatinine | Not Specified | Not Specified | Normal | | details | | Urine | Detected and Quantified | 0-2.0 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | 0.15-5.69 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | 1.2-4.3 umol/mmol creatinine | Infant (0-1 year old) | Female | Normal | | details | | Urine | Detected and Quantified | 0.80-1.8 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 20-50 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.7 (0.2-1.7) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 1.948-11.689 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected and Quantified | <25 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | <97.405 umol/mmol creatinine | Infant (0-1 year old) | Male | Normal | | details | | Urine | Detected and Quantified | 1.900-8.600 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.4 (0.2-0.8) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <10.25 umol/mmol creatinine | Children (1 - 18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 10.34 (6.40-21.12) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.1-0.4 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.75-1.2 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 0.1 (0.1-1.7) umol/mmol creatinine | Children (1-13 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 1.342 +/- 1.317 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 0.2 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <7 umol/mmol creatinine | Children (1-13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 0.95 +/- 0.93 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 2.09-37.66 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 10.4 (2.8-53.7) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 15,80 +/- 11.61 umol/mmol creatinine | Newborn (0-30 days old) | Female | Normal | | details | | Urine | Detected and Quantified | 13.27 +/- 7.27 umol/mmol creatinine | Newborn (0-30 days old) | Male | Normal | | details | | Urine | Detected and Quantified | 0.5 (0.1-1.7) umol/mmol creatinine | Adolescent (13-18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 10.7 (0.1-28.2) umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected and Quantified | 2-40 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 11.0 (2.00-20.0) uM | Elderly (>65 years old) | Both | Alzheimer's disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | ankylosing spondylitis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | rheumatoid arthritis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Irritable bowel syndrome | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | asymptomatic diverticulosis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | symptomatic uncomplicated diverticular disease | | details | | Urine | Detected and Quantified | 17-124 umol/mmol creatinine | Infant (0-1 year old) | Male | 2-Ketoglutarate dehydrogenase complex deficiency | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected and Quantified | 0-14.611 umol/mmol creatinine | Infant (0-1 year old) | Both | Amish lethal microcephaly | | details | | Urine | Detected and Quantified | 482.3 umol/mmol creatinine | Infant (0-1 year old) | Male | Fumarase deficiency | | details | | Urine | Detected and Quantified | 2269.534 umol/mmol creatinine | Infant (0-1 year old) | Male | Fumarase deficiency | | details | | Urine | Detected and Quantified | 151-772 umol/mmol creatinine | Adult (>18 years old) | Both | Fumaric aciduria | | details | | Urine | Detected and Quantified | 217-445 umol/mmol creatinine | Children (1 - 13 years old) | Female | Fumaric aciduria | | details | | Urine | Detected and Quantified | 313.400-493.200 umol/mmol creatinine | Infant (0-1 year old) | Male | Fumarase deficiency | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected and Quantified | 0.7 (0.0-4.0) umol/mmol creatinine | Adult (>18 years old) | Both | Lung cancer | | details | | Urine | Detected and Quantified | 11.39 +/- 28.464 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected and Quantified | 48 umol/mmol creatinine | Children (1-13 years old) | Male | Lipoyltransferase 1 Deficiency | | details |
| Show more...
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Colorectal cancer |
|---|
- Ni Y, Xie G, Jia W: Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J Proteome Res. 2014 Sep 5;13(9):3857-70. doi: 10.1021/pr500443c. Epub 2014 Aug 14. [PubMed:25105552 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Alzheimer's disease |
|---|
- Redjems-Bennani N, Jeandel C, Lefebvre E, Blain H, Vidailhet M, Gueant JL: Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology. 1998;44(5):300-4. [PubMed:9693263 ]
| | Irritable bowel syndrome |
|---|
- Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH: Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011 Jun;60(Pt 6):817-27. doi: 10.1099/jmm.0.028126-0. Epub 2011 Feb 17. [PubMed:21330412 ]
| | Diverticular disease |
|---|
- Tursi A, Mastromarino P, Capobianco D, Elisei W, Miccheli A, Capuani G, Tomassini A, Campagna G, Picchio M, Giorgetti G, Fabiocchi F, Brandimarte G: Assessment of Fecal Microbiota and Fecal Metabolome in Symptomatic Uncomplicated Diverticular Disease of the Colon. J Clin Gastroenterol. 2016 Oct;50 Suppl 1:S9-S12. doi: 10.1097/MCG.0000000000000626. [PubMed:27622378 ]
| | Rheumatoid arthritis |
|---|
- Tie-juan ShaoZhi-xing HeZhi-jun XieHai-chang LiMei-jiao WangCheng-ping Wen. Characterization of ankylosing spondylitis and rheumatoid arthritis using 1H NMR-based metabolomics of human fecal extracts. Metabolomics. April 2016, 12:70 [Link]
| | Lung Cancer |
|---|
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I: HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009 Jan;37(Database issue):D603-10. doi: 10.1093/nar/gkn810. Epub 2008 Oct 25. [PubMed:18953024 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
| | Fumarase deficiency |
|---|
- Tregoning S, Salter W, Thorburn DR, Durkie M, Panayi M, Wu JY, Easterbrook A, Coman DJ: Fumarase deficiency in dichorionic diamniotic twins. Twin Res Hum Genet. 2013 Dec;16(6):1117-20. doi: 10.1017/thg.2013.72. Epub 2013 Nov 4. [PubMed:24182348 ]
- Bastug O, Kardas F, Ozturk MA, Halis H, Memur S, Korkmaz L, Tag Z, Gunes T: A rare cause of opistotonus; fumaric aciduria: The first case presentation in Turkey. Turk Pediatri Ars. 2014 Mar 1;49(1):74-6. doi: 10.5152/tpa.2014.442. eCollection 2014 Mar. [PubMed:26078636 ]
- Whelan DT, Hill RE, McClorry S: Fumaric aciduria: a new organic aciduria, associated with mental retardation and speech impairment. Clin Chim Acta. 1983 Aug 31;132(3):301-8. [PubMed:6616883 ]
- Maradin M, Fumic K, Hansikova H, Tesarova M, Wenchich L, Dorner S, Sarnavka V, Zeman J, Baric I: Fumaric aciduria: mild phenotype in a 8-year-old girl with novel mutations. J Inherit Metab Dis. 2006 Oct;29(5):683. Epub 2006 Aug 5. [PubMed:16972175 ]
- G.Frauendienst-Egger, Friedrich K. Trefz (2017). MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de). METAGENE consortium.
| | 2-Ketoglutarate dehydrogenase complex deficiency |
|---|
- Bonnefont JP, Chretien D, Rustin P, Robinson B, Vassault A, Aupetit J, Charpentier C, Rabier D, Saudubray JM, Munnich A: Alpha-ketoglutarate dehydrogenase deficiency presenting as congenital lactic acidosis. J Pediatr. 1992 Aug;121(2):255-8. [PubMed:1640293 ]
| | Amish lethal microcephaly |
|---|
- Kelley RI, Robinson D, Puffenberger EG, Strauss KA, Morton DH: Amish lethal microcephaly: a new metabolic disorder with severe congenital microcephaly and 2-ketoglutaric aciduria. Am J Med Genet. 2002 Nov 1;112(4):318-26. doi: 10.1002/ajmg.10529. [PubMed:12376931 ]
| | Lipoyltransferase 1 Deficiency |
|---|
- Soreze Y, Boutron A, Habarou F, Barnerias C, Nonnenmacher L, Delpech H, Mamoune A, Chretien D, Hubert L, Bole-Feysot C, Nitschke P, Correia I, Sardet C, Boddaert N, Hamel Y, Delahodde A, Ottolenghi C, de Lonlay P: Mutations in human lipoyltransferase gene LIPT1 cause a Leigh disease with secondary deficiency for pyruvate and alpha-ketoglutarate dehydrogenase. Orphanet J Rare Dis. 2013 Dec 17;8:192. doi: 10.1186/1750-1172-8-192. [PubMed:24341803 ]
|
|
|---|
| Associated OMIM IDs | - 114500 (Colorectal cancer)
- 104300 (Alzheimer's disease)
- 180300 (Rheumatoid arthritis)
- 211980 (Lung Cancer)
- 610247 (Eosinophilic esophagitis)
- 606812 (Fumarase deficiency)
- 203740 (2-Ketoglutarate dehydrogenase complex deficiency)
- 607196 (Amish lethal microcephaly)
- 616299 (Lipoyltransferase 1 Deficiency)
|
|---|
| External Links |
|---|
| DrugBank ID | DB01677 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB003291 |
|---|
| KNApSAcK ID | C00001183 |
|---|
| Chemspider ID | 10197150 |
|---|
| KEGG Compound ID | C00122 |
|---|
| BioCyc ID | FUM |
|---|
| BiGG ID | 33938 |
|---|
| Wikipedia Link | Fumaric_Acid |
|---|
| METLIN ID | 3242 |
|---|
| PubChem Compound | 444972 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 18012 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | FUM |
|---|
| MarkerDB ID | MDB00000065 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Dong, Changsheng; Ma, Xinming. Method for preparation of fumaric acid from the tail gas acid spray solution from oxidation of phthalic anhydride. Faming Zhuanli Shenqing Gongkai Shuomingshu (2007), 5pp. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [PubMed:2026685 ]
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. [PubMed:8087979 ]
- Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL: Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648-69. [PubMed:8412012 ]
- Redjems-Bennani N, Jeandel C, Lefebvre E, Blain H, Vidailhet M, Gueant JL: Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology. 1998;44(5):300-4. [PubMed:9693263 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|