| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:07 UTC |
|---|
| HMDB ID | HMDB0000896 |
|---|
| Secondary Accession Numbers | - HMDB0004011
- HMDB00896
- HMDB04011
|
|---|
| Metabolite Identification |
|---|
| Common Name | Taurodeoxycholic acid |
|---|
| Description | Taurodeoxycholic acid is a bile salt formed in the liver by conjugation of deoxycholate with taurine, usually as the sodium salt. Bile acids are steroid acids found predominantly in the bile of mammals. The distinction between different bile acids is minute, depending only on the presence or absence of hydroxyl groups on positions 3, 7, and 12. Bile acids are physiological detergents that facilitate excretion, absorption, and transport of fats and sterols in the intestine and liver. Bile acids are also steroidal amphipathic molecules derived from the catabolism of cholesterol. They modulate bile flow and lipid secretion, are essential for the absorption of dietary fats and vitamins, and have been implicated in the regulation of all the key enzymes involved in cholesterol homeostasis. Bile acids recirculate through the liver, bile ducts, small intestine and portal vein to form an enterohepatic circuit. They exist as anions at physiological pH and, consequently, require a carrier for transport across the membranes of the enterohepatic tissues. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients. Bile acids have potent toxic properties (e.g. membrane disruption) and there are a plethora of mechanisms to limit their accumulation in blood and tissues (PMID:11316487 , 16037564 , 12576301 , 11907135 ). Taurodeoxycholic acid can be found in Escherichia (PMID:30736766 ). |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)NCCS(O)(=O)=O InChI=1S/C26H45NO6S/c1-16(4-9-24(30)27-12-13-34(31,32)33)20-7-8-21-19-6-5-17-14-18(28)10-11-25(17,2)22(19)15-23(29)26(20,21)3/h16-23,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17-,18-,19+,20-,21+,22+,23+,25+,26-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Taurodeoxycholate | ChEBI | | Acid, taurodeoxycholic | HMDB | | Deoxycholate, taurine | HMDB | | Sodium taurodeoxycholate | HMDB | | Taurine deoxycholate | HMDB | | Deoxycholyltaurine | HMDB | | Taurodeoxycholate, sodium | HMDB | | Deoxytaurocholate | HMDB | | Deoxytaurocholic acid | HMDB | | N-(3a,12a-Dihydroxy-5b-cholan-24-oyl)-taurine | HMDB | | Sodium taurodeoxylate | HMDB | | Taurodeoxycholic acid sodium salt | HMDB | | Taurodeoxycholic acid sodium salt hydrate | HMDB | | Taurodesoxycholate | HMDB | | Taurodesoxycholic acid | HMDB | | Tudcabil | HMDB |
|
|---|
| Chemical Formula | C26H45NO6S |
|---|
| Average Molecular Weight | 499.704 |
|---|
| Monoisotopic Molecular Weight | 499.296758867 |
|---|
| IUPAC Name | 2-[(4R)-4-[(1S,2S,5R,7R,10R,11S,14R,15R,16S)-5,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethane-1-sulfonic acid |
|---|
| Traditional Name | 2-[(4R)-4-[(1S,2S,5R,7R,10R,11S,14R,15R,16S)-5,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethanesulfonic acid |
|---|
| CAS Registry Number | 516-50-7 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)NCCS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C26H45NO6S/c1-16(4-9-24(30)27-12-13-34(31,32)33)20-7-8-21-19-6-5-17-14-18(28)10-11-25(17,2)22(19)15-23(29)26(20,21)3/h16-23,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17-,18-,19+,20-,21+,22+,23+,25+,26-/m1/s1 |
|---|
| InChI Key | AWDRATDZQPNJFN-VAYUFCLWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Taurinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taurinated bile acid
- Dihydroxy bile acid, alcohol, or derivatives
- Hydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 12-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- Fatty amide
- Fatty acyl
- N-acyl-amine
- Cyclic alcohol
- Alkanesulfonic acid
- Sulfonyl
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organosulfur compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 204 - 208 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 41 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | | Show more...
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Taurodeoxycholic acid,1TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4275.2 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4209.0 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TMS,isomer #3 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4327.9 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4261.9 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4140.3 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4322.3 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4240.1 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #4 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4250.7 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #5 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4156.1 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TMS,isomer #6 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4311.1 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4185.6 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4429.9 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4824.8 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4073.0 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4491.7 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4889.7 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4256.7 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4504.4 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4844.2 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4198.5 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4509.3 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4795.8 | Standard polar | 33892256 | | Taurodeoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4130.8 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4644.9 | Standard non polar | 33892256 | | Taurodeoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C)[C@@]21C | 4650.8 | Standard polar | 33892256 | | Taurodeoxycholic acid,1TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4474.7 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TBDMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4401.0 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TBDMS,isomer #3 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4513.4 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,1TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4517.3 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4529.5 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4736.8 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4686.8 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #4 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4640.9 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #5 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4601.5 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,2TBDMS,isomer #6 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4755.6 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4759.7 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 5209.0 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4920.1 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4738.3 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 5216.7 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4988.9 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4907.7 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 5277.7 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3C[C@H](O)[C@@]21C | 4948.3 | Standard polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4851.3 | Semi standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 5288.8 | Standard non polar | 33892256 | | Taurodeoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C | 4907.6 | Standard polar | 33892256 |
| Show more...
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lu-0111900000-a90e31e3c4cf2f0c6f3a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (2 TMS) - 70eV, Positive | splash10-004i-4201229000-01b8d5e2d286a6e403aa | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurodeoxycholic acid GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0ikd-0047930000-8f9f65d8c1d3c8253501 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-03g0-0950300000-d3a559bd8c5ccd049d7b | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-001i-9545740000-d9a7750b1b579bd2bb05 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid LC-ESI-IT , negative-QTOF | splash10-0a4i-0009500000-11f56619a58a7f9fe2df | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid 10V, Positive-QTOF | splash10-0udi-0000690000-02978b9c502ebd7d68d6 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid 20V, Positive-QTOF | splash10-03di-0000900000-c7214ee029f71204bb8f | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Positive-QTOF | splash10-0470-1596200000-357f5937f8ae2d422ba2 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Positive-QTOF | splash10-0470-1596200000-078d7c7763da8bb11d2e | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 10V, Positive-QTOF | splash10-01si-0302920000-c2629184642c876303ac | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 20V, Positive-QTOF | splash10-0a6r-1904400000-52b97ee82fecb26d2df7 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Positive-QTOF | splash10-004l-8906300000-1c0dd8ea92d737243ce2 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 10V, Negative-QTOF | splash10-000t-2001900000-5090bd6cae3b061c80e4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 20V, Negative-QTOF | splash10-0089-7504900000-0d93eba8b9f15618357f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Negative-QTOF | splash10-001l-9202000000-2e68ed7e43eb97ec4ad8 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 10V, Negative-QTOF | splash10-0002-0000900000-31f17d1d48b7afdf3bae | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 20V, Negative-QTOF | splash10-0002-0000900000-09a127bb8eacbd0a6ab7 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Negative-QTOF | splash10-001j-9201800000-97168e49aaa085a3735c | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 10V, Positive-QTOF | splash10-0f89-0000950000-e77d9cab9a8afc22e8b1 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 20V, Positive-QTOF | splash10-08gi-2414910000-42610292496f161b3e8d | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurodeoxycholic acid 40V, Positive-QTOF | splash10-052e-8912000000-fd93247f3ff2a43d83dd | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
| Show more...
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | |
|---|
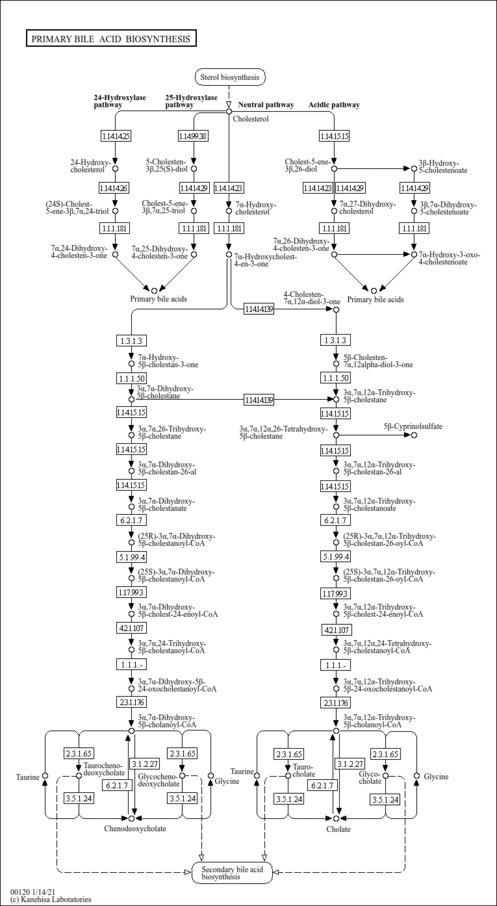
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Bile | Detected and Quantified | > 0.01 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.062 (0.000-0.177) uM | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected and Quantified | 4.32 +/- 5.81 nmol/g dry feces | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
| Show more...
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Hepatocellular carcinoma | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Liver Cirrhosis | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Metastatic melanoma | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Hepatocellular carcinoma |
|---|
- Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, Cheema AK, Tadesse MG, Goldman R, Shetty K: Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012 Sep 19;743:90-100. doi: 10.1016/j.aca.2012.07.013. Epub 2012 Jul 20. [PubMed:22882828 ]
| | Cirrhosis |
|---|
- Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, Cheema AK, Tadesse MG, Goldman R, Shetty K: Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012 Sep 19;743:90-100. doi: 10.1016/j.aca.2012.07.013. Epub 2012 Jul 20. [PubMed:22882828 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
| | Metastatic melanoma |
|---|
- Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY: Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia. 2017 Oct;19(10):848-855. doi: 10.1016/j.neo.2017.08.004. Epub 2017 Sep 15. [PubMed:28923537 ]
|
|
|---|
| Associated OMIM IDs | - 114550 (Hepatocellular carcinoma)
- 114500 (Colorectal cancer)
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022304 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 2015539 |
|---|
| KEGG Compound ID | C05463 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Taurodeoxycholic_acid |
|---|
| METLIN ID | 5853 |
|---|
| PubChem Compound | 2733768 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 9410 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | TDECHOLA |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Parenti, Massimo. Preparation of tauroursodesoxycholic acid dihydrate. Eur. Pat. Appl. (1990), 3 pp. CODEN: EPXXDW EP 400695 A2 19901205 CAN 114:82269 AN 1991:82269 |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Bloch CA, Watkins JB: Determination of conjugated bile acids in human bile and duodenal fluid by reverse-phase high-performance liquid chromatography. J Lipid Res. 1978 May;19(4):510-3. [PubMed:659989 ]
- Foley DP, Collins BR, Magee JC, Platt JL, Katz E, Harland RC, Meyers WC, Chari RS: Bile acids in xenogeneic ex-vivo liver perfusion: function of xenoperfused livers and compatibility with human bile salts and porcine livers. Transplantation. 2000 Jan 27;69(2):242-8. [PubMed:10670634 ]
- Bretagne JF, Vidon N, L'Hirondel C, Bernier JJ: Increased cell loss in the human jejunum induced by laxatives (ricinoleic acid, dioctyl sodium sulphosuccinate, magnesium sulphate, bile salts). Gut. 1981 Apr;22(4):264-9. [PubMed:6165655 ]
- Leveau P, Wang X, Sun Z, Borjesson A, Andersson E, Andersson R: Severity of pancreatitis-associated gut barrier dysfunction is reduced following treatment with the PAF inhibitor lexipafant. Biochem Pharmacol. 2005 May 1;69(9):1325-31. [PubMed:15826603 ]
- Burkard I, von Eckardstein A, Rentsch KM: Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Nov 5;826(1-2):147-59. Epub 2005 Sep 22. [PubMed:16182619 ]
- Lim HJ, Kim SY, Lee WK: Isolation of cholesterol-lowering lactic acid bacteria from human intestine for probiotic use. J Vet Sci. 2004 Dec;5(4):391-5. [PubMed:15613825 ]
- Muhlbauer M, Allard B, Bosserhoff AK, Kiessling S, Herfarth H, Rogler G, Scholmerich J, Jobin C, Hellerbrand C: Differential effects of deoxycholic acid and taurodeoxycholic acid on NF-kappa B signal transduction and IL-8 gene expression in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004 Jun;286(6):G1000-8. Epub 2004 Jan 15. [PubMed:14726307 ]
- Pouwels MJ, Tack CJ, Span PN, Olthaar AJ, Sweep CG, Huvers FC, Lutterman JA, Hermus AR: Role of hexosamines in insulin resistance and nutrient sensing in human adipose and muscle tissue. J Clin Endocrinol Metab. 2004 Oct;89(10):5132-7. [PubMed:15472217 ]
- Elkins CA, Mullis LB: Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl Environ Microbiol. 2004 Dec;70(12):7200-9. [PubMed:15574918 ]
- Aubert E, Sbarra V, Le Petit-Thevenin J, Valette A, Lombardo D: Site-directed mutagenesis of the basic N-terminal cluster of pancreatic bile salt-dependent lipase. Functional significance. J Biol Chem. 2002 Sep 20;277(38):34987-96. Epub 2002 Jul 10. [PubMed:12110666 ]
- Xie Q, Li GM, Zhou XQ, Liao D, Yu H, Guo Q: [Effect of Tauroursodeoxycholic acid on cytochrome C-mediated apoptosis in HepG2 cells]. Zhonghua Gan Zang Bing Za Zhi. 2003 May;11(5):298-301. [PubMed:12773247 ]
- St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ: Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001 May;204(Pt 10):1673-86. [PubMed:11316487 ]
- Claudel T, Staels B, Kuipers F: The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2020-30. Epub 2005 Jul 21. [PubMed:16037564 ]
- Chiang JY: Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003 Mar;284(3):G349-56. [PubMed:12576301 ]
- Davis RA, Miyake JH, Hui TY, Spann NJ: Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J Lipid Res. 2002 Apr;43(4):533-43. [PubMed:11907135 ]
- Xu Y, Yang L, Zhao S, Wang Z: Large-scale production of tauroursodeoxycholic acid products through fermentation optimization of engineered Escherichia coli cell factory. Microb Cell Fact. 2019 Feb 8;18(1):34. doi: 10.1186/s12934-019-1076-2. [PubMed:30736766 ]
| Show more...
|---|