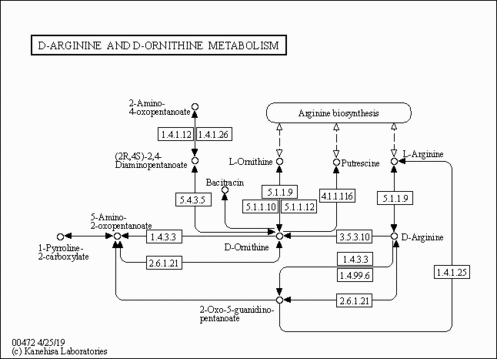
| 5-Amino-2-oxopentanoic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=O)CCCN | 1324.8 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=CCCN)C(=O)O | 1430.0 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,1TMS,isomer #3 | C[Si](C)(C)NCCCC(=O)C(=O)O | 1451.8 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C | 1479.6 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C | 1483.8 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C | 2015.7 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #2 | C[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C | 1502.3 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #2 | C[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C | 1562.2 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #2 | C[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C | 1692.5 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #3 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O | 1619.3 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #3 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O | 1679.7 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #3 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O | 1891.6 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #4 | C[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C | 1692.9 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #4 | C[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C | 1610.3 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TMS,isomer #4 | C[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C | 1993.2 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #1 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1630.9 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #1 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1658.8 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #1 | C[Si](C)(C)NCCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1646.2 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C)[Si](C)(C)C | 1737.4 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C)[Si](C)(C)C | 1657.3 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C)[Si](C)(C)C | 1673.6 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=CCCN([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 1813.5 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=CCCN([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 1779.6 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=CCCN([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 1842.6 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C)[Si](C)(C)C)O[Si](C)(C)C | 1786.2 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C)[Si](C)(C)C)O[Si](C)(C)C | 1750.2 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C)[Si](C)(C)C)O[Si](C)(C)C | 1623.7 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=O)CCCN | 1565.4 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=CCCN)C(=O)O | 1674.3 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NCCCC(=O)C(=O)O | 1720.0 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C(C)(C)C | 1953.2 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C(C)(C)C | 1848.4 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN)O[Si](C)(C)C(C)(C)C | 2119.9 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C(C)(C)C | 1979.2 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C(C)(C)C | 1931.7 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NCCCC(=O)C(=O)O[Si](C)(C)C(C)(C)C | 1940.5 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O | 2074.9 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O | 2023.5 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O | 2066.6 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C(C)(C)C | 2142.2 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C(C)(C)C | 2015.9 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(CCCC(=O)C(=O)O)[Si](C)(C)C(C)(C)C | 2092.5 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2274.0 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2155.3 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2045.0 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2378.5 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2253.2 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(=O)CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2055.3 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2447.1 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2309.0 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2151.2 | Standard polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2640.8 | Semi standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2420.8 | Standard non polar | 33892256 |
| 5-Amino-2-oxopentanoic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(=CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2110.0 | Standard polar | 33892256 |